Once computational modeling and simulation (CM&S) was officially sanctioned by the FDA, “regulatory grade analysis” has become a rapidly growing trend in medical device development. SES Force-4’s leveraging of computational modeling and simulation based approaches throughout the product lifecycle predates the FDA acceptance of CM&S technologies by decades and includes supporting clinical and non-clinical activities in the medical industry.

Computational modeling of the intraocular lens (IOL) delivery into the eye during cataract surgery
The growing reliance on CM&S to provide scientific evidence for regulatory submissions is expected to continue with the introduction of the ASME V&V 40 standard (2018) and the guidance provided by the FDA (2023). The Center for Devices and Radiological Health (CDRH) has identified ‘Computational Modeling Technologies to Support Regulatory Decision-making’ as one of its regulatory science priorities.
SES has developed an internal procedure for building and executing regulatory grade computational models, which uses a risk-informed approach to assess the model credibility under the framework provided by the ASME V&V 40 standard. SES has had multiple successful regulatory submissions to the FDA and EU MDR using CM&S based approaches for performing in silico design verification testing of medical devices.
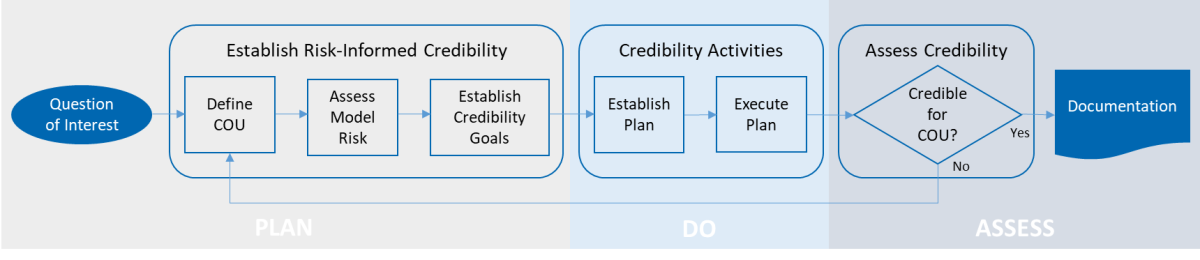
Overview of SES’ risk informed credibility assessment process for computational model (adapted based on the framework provided by the ASME V&V 40-2018 standard)
Advantages of in silico Design Verification Testing
Using computational modeling and simulation (CM&S) based approaches for in silico design verification testing of medical devices offers several advantages over traditional physical testing methods.
1. Cost and Time Efficiency:
Stresses in the human femur during a walking step
CM&S allows for virtual testing, eliminating the need for expensive and time-consuming physical prototypes. Iterative design changes can be quickly evaluated through simulations, reducing the overall development time and cost.
Medical devices, especially in applications involving high patient risk, have elevated requirements of reliability and accuracy, which in most cases entails evaluation at worst-case assembly tolerance conditions. Conducting physical testing involves building and assembling components with various tolerance combinations to obtain assemblies at worst-case tolerance conditions. This can be logistically challenging and resource-intensive and in some cases, not possible. (image from brochure 513)
Computational modeling and simulation offer an efficient and cost-effective alternative to physical testing. Through simulation, variations in component dimensions and properties can be systematically modeled and analyzed, encompassing a wide range of tolerance combinations. Simulations enable the exploration of extreme scenarios that may be difficult to replicate in physical experiments, providing insights into potential failure modes or performance limitations.
2. Enhanced Understanding, Flexibility and Adaptability
Computational models offer a deeper understanding of the underlying physics and mechanisms governing device behavior, aiding in design optimization and troubleshooting. Visualization tools allow stakeholders to observe and analyze complex phenomena that may not be easily observable in physical experiments.
Well-validated computational models can accurately predict device performance under various conditions, providing valuable insights into its behavior. Virtual testing enables the assessment of device performance across a broader range of parameters than what may be feasible with physical testing alone. CM&S provides flexibility in testing different design iterations, materials, and configurations without the need for significant retooling or setup changes. Models can be easily updated to incorporate new data, insights, or design modifications, allowing for rapid adaptation to changing requirements or regulatory feedback. (Image from brochure 326)
3. Time-to-market and Competitive Advantage
The use of in silico design verification testing can significantly improve the time to market and provide a competitive advantage for medical device manufacturer through accelerated design iterations, reduced development costs, improved regulatory compliance and enhanced product quality and performance.
Deeper understanding of the underlying physics of the problem through computational modeling provides a platform for bringing innovative and improved products to the market faster. Shorter development cycles facilitated by in silico testing enable manufacturers to respond more rapidly to changing market demands and emerging trends.
4. Risk Reduction:
By conducting virtual testing, potential risks associated with physical testing, such as equipment failure or safety hazards, can be minimized. CM&S facilitates the exploration of a wide range of design scenarios and operating conditions, helping identify and mitigate risks early in the development process.
5. Regulatory Compliance:
The guidance provided by the FDA for ‘Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions’ (2023) provides a structured framework for the verification and validation of computational models, ensuring their reliability and trustworthiness. SES’ internal procedure leverages the FDA guidance and the ASME V&V 40 standard and is designed to help manufacturers demonstrate compliance with regulatory requirements for the use of CM&S in design verification testing of medical devices.
With the internal V&V 40 procedure included in SES’s quality system and multiple successful regulatory submissions, SES is uniquely positioned to leverage its core competencies in CM&S to lead the medical device industry in the use of regulatory grade computational models for in silico design verification testing.

Author
Keep in touch with us.
Sign up for our newsletter.



