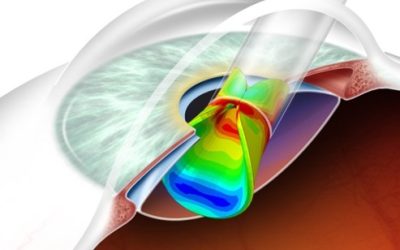
In the pharmaceutical industry, Container Closure Integrity (CCI) stands as a linchpin, ensuring the safety, efficacy, and sterility of parenteral packaging. A secure seal is not just a technicality; it’s the assurance that drugs reach patients in their intended state, free from contamination, and with therapeutic properties intact.
This blog sheds light on the increasing and evolving challenges surrounding CCI, emphasizing the pressing need for a more sophisticated, scientific understanding and empirical demonstration as the safety factor dwindles.
Challenges: Novel Pharmaceuticals and Antiquated Containers
The pharmaceutical industry has historically employed archaic methods to guarantee a reliable seal. This involved over-compressing a ‘squishy and thick’ seal and extensive testing of a high quantity of specimens to establish statistical confidence. However, the industry is now at the cusp as significant challenges and new use cases are being required.
1.Emergence of New Drug Classes: The advent of novel drug therapies, exemplified by biologics and mRNA-based vaccines, has revolutionized the storage requirements. These cutting-edge formulations often mandate ultra-low temperatures or material limitations, posing a formidable challenge to the conventional sealing mechanisms used in traditional stoppers.
2. Cold Storage Conditions: The pharmaceutical landscape is witnessing an expansion in the range of storage temperatures and handling scenarios. This diversification necessitates packaging solutions that are not only adaptable but also resilient in the face of varying conditions, further complicating the assurance of container closure integrity.
3. Advances in Materials: In response to the evolving landscape, material selection has seen remarkable advances. The introduction of cyclic olefin copolymers (COCs), fluoropolymers, innovative films/coatings, and lubrication methods offer enhanced properties with specific performance advantages for stability, injection performance, or manufacturability. However, they also presented new challenges for CCI. With their distinct characteristics, these materials demand a nuanced understanding of their behavior in the context of seal integrity.
4. Novel Delivery Devices: The evolution of drug delivery devices, including autoinjectors and prefilled syringes, has added a layer of complexity to CCI considerations. These innovative systems introduce unique challenges that necessitate a comprehensive understanding of the physics of sealing, especially in the context of these novel delivery mechanisms.
5. Stringent Regulatory Expectations: Regulatory agencies are increasingly heightening their scrutiny of CCI testing methodologies. There is a growing demand for more comprehensive data to substantiate product safety claims, placing additional pressure on pharmaceutical companies to enhance their understanding of the physics underlying seal integrity.
6. ‘Last Mile’ Shipment: The shift towards delivering pharmaceutical products in saleable boxes directly to patients’ homes introduces new challenges in maintaining CCI. The varied environmental conditions, stacking, and handling during transit and storage demand innovative packaging solutions to ensure product safety.
7. Sustainability Challenges: The move towards sustainable practices in pharmaceutical packaging introduces challenges related to material choices and design. Balancing sustainability with the stringent requirements of container closure integrity adds a layer of complexity, necessitating careful consideration of environmentally friendly alternatives without compromising the seal’s reliability.
8. PFAS: The proposed restriction of PFASs in the European Union and the associated environmental, human, and animal health consequences present a formidable challenge to the pharmaceutical industry. Coatings in primary packaging, such as container-closure systems, often contain PFAS, and the industry must now grapple with the potential impact of the proposed ban on these substances, considering not only regulatory compliance but also sustainability and environmental responsibility.
This confluence of new factors is undeniably shrinking design margins, necessitating a shift towards a more robust, physics-based understanding to guide testing practices and establish reliability limits. As the industry navigates these evolving challenges, the imperative is clear: a proactive embrace of cutting-edge scientific principles, including advancements in material selection and lubrication methods, is required to ensure the continued reliability and safety of pharmaceutical packaging. By marrying empirical testing with a deeper comprehension of the underlying physics, pharmaceutical companies can fortify their approaches, addressing the intricate demands imposed by these emerging factors on container closure integrity.
Explore the SES Case Studies for additional discussion on these obstacles.

AUTHOR
Jeremy Hemmingway, P.E., Head of Drug Delivery Solutions

Reference: The Potential for Chronopharmacology to Revolutionize Patient Outcomes – B013-PDD
Keep in touch with us. Sign up for our newsletter.