Keep in touch with us.
Sign up for our newsletter.
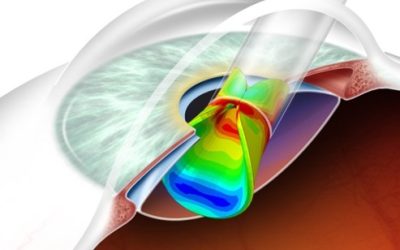
In Silico Design Verification Testing of Medical Devices
Once computational modeling and simulation (CM&S) was officially sanctioned by the FDA, “regulatory grade analysis” has become a rapidly growing trend in medical device development. SES Force-4’s leveraging of computational modeling and simulation based approaches...