THE PROJECT
Force-4/SES Team shares the Gates Foundation’s future vision of a world where high-value pharmaceuticals in healthcare are made more available globally with reduced cost.
“The most effective way to improve outcomes in this environment of scarcity is to generate “more health for the money” by improving health system performance, developing higher-impact tools and solutions, and building robust markets and distribution channels for crucial health commodities.” The Bill & Melinda Gates Foundation
THE CHALLENGE
Working toward this vision, Force-4 developed FLEXURE, a dual chamber device that uniquely solves the challenges of making an injector that is low cost and available for use worldwide. This approach aligns well with several Gates Foundation key vision areas:
- Low Cost
- Efficacy In Drug Delivery
- Broad Usability, Safety, And Sustainability
Low Cost
Force-4’s device development approach for FLEXURE utilizes low-cost / high-volume packaging approaches to form a dual chamber injection device. This approach ultimately minimizes the cost of the injector at high volumes. The impact of this is an effective, low-cost device that is easily operated and available globally.
Efficacy In Drug Delivery
The Flexure devices provide the following benefits:
- Compact
- Material Efficiency
- Light Weight
- Ruggedness
- Accurate and Consistent Reconstitution
- Low Cost
From a human performance standpoint, our approach is an intuitive, user-centered design aimed at holistic compliance. This is accomplished for the one administering the medication by minimizing steps and making usage fail-safe. For the patient, the design seeks to convey an impression of safety, approachability and total efficacy.
Broad Usability, Safety, and Sustainability
Force-4/SES team designed a dual chamber injector device that is easy to use with minimal instruction or learned behavior. It is completely reliable in drug reconstitution and simply and easily delivers the drug. Effortless to use, it is a distinctive device having a uniquely intuitive operation that can serve as an iconic symbol of advancement in efforts to improve world health.
THE SOLUTION
The design of FLEXURE is relatively simple in construction. It utilizes known technologies from high volume packaging, which drive reliability and sustainability and reduce cost.
Design Concept
The sealing and frangible membrane features are the core of the engineering development work. While the cost of components is low, the biggest challenge is the performance of the materials and the special mechanical properties joining them together. The design of bulkheads forming the dual chambers are specially designed for enhanced mixing. The sterile fluid is injected into the bulkhead containing a lyophilized tablet. The structure of the flow paths has been designed to create a swirling ‘wave’ to aid the reconstitution of the drug. After mixing, activation of the final fold creates an injector ready for single use.
Flexure technology utilizes welded and bonded films to create individual chambers for housing and mixing liquid and dry mediums. This approach is similar to blister-type packaging used in many over-the-counter (OTC) medications.
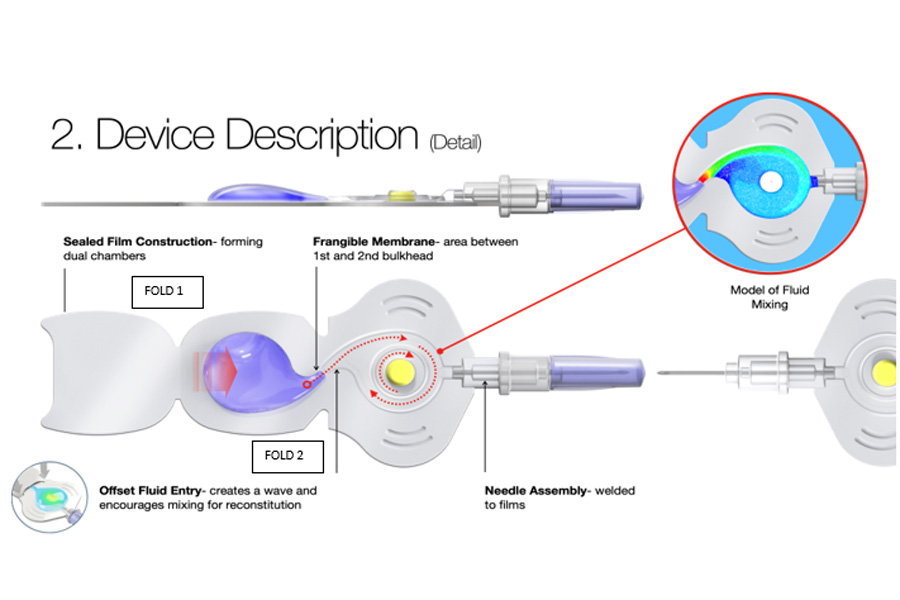
Design Details
There are two chambers in the FLEXURE design — one for the sterile diluent and the other for a lyophilized tablet containing the active drug. The second chamber is also used as a mixing bulkhead to prepare the reconstitution, with specially designed features for reconstitution and mixing. A frangible membrane separates the dual chambers. The frangible membrane ruptures when the device is folded and squeezed membrane. The frangible membrane ruptures when the device is folded and squeezed.
The FLEXURE device requires folds in a five-step process to prepare the injector for use (refer to Figure 2). Device Description:
- The first fold increases pressure in the first liquid chamber, causing the frangible membrane between chambers to release liquid into the second chamber, including a lyophilized tablet containing the pharmaceutical.
- Once the panel is completely folded and squeezed flat, the user knows that the first chamber is fully evacuated.
- After sufficient time for mixing and slight agitation, the medication is ready for injection. This is initiated through the second fold. By folding the device nearly in half, pressure is increased in the mixing bulkhead.
- When the needle cap is twisted, the proximal end of the needle is introduced into the mixing bulkhead. Once the needle cap is removed, the medication is ready to be administered.
- The folded construction forms a tool actuated between the thumb and fingers. This tool is intended to provide control and positive tactile feedback while administering the medication.
By design, FLEXURE has very low material mass. This translates into less overall medical waste and is a positive step to making single-use injection devices more sustainable, both in the amount of material needed in the device and the energy it takes to produce and transport.
THE IMPACT
Force-4’s/SES’s design approach centers on minimizing operations to eliminate user errors. FLEXURE is sequential in use — it cannot be reversed or repurposed for another injection. This all supports a design whose operation is easy to understand and intuitive to operate. FLEXURE is a clear approach to a dual chamber solution combined with its minimal form factor and low material mass.
Force-4/SES device development approach produced a dual chamber device for injection that is easy to operate, made of low-cost/high-volume packaging material, and suitable for global transport.
SUCCESS!
In the future, injectors like FLEXURE will be able to make life-saving and life-enhancing medications more accessible to more people throughout the world. This is especially true in geographies and environments where medications are difficult to transport or access to medical care is scarce. Access to medicine and the ability to easily and safely administer it is essential to global needs.
At Force-4, Smart Ideas For A Smart World, we are a passionate, multidisciplinary team of expert problem-solvers ready to tackle your most challenging technical problems. We pride ourselves on our innovative solutions, which elevate how businesses operate. Partnering with SES has provided forward-thinking solutions for companies and industries requiring in-depth technical knowledge and proven performance in engineering design and analysis, thermal and fluid sciences, instrumentation, and testing.
More about Force–4 & SES Team… Force-4 and its engineering parent company, SES (Stress Engineering Services, Inc.), have 50 years of experience developing and deploying proprietary predictive analysis methods that accelerate the development process while reducing development risk. Our human-centered and design-by-analysis approach eliminates trial and error rework and enables design optimization before it is necessary to commit to tooling.
AUTHORS

- Jason Phillips, Group Creative Director, has more than 20 years of innovation and product development experience in consumer-packaged goods, durable products, human performance applications, and technology integration.
Ref: C003-PDD-Innovative solutions for the Gates Foundation dual chamber pharma injection device
Keep in touch with us.
Sign up for our newsletter.